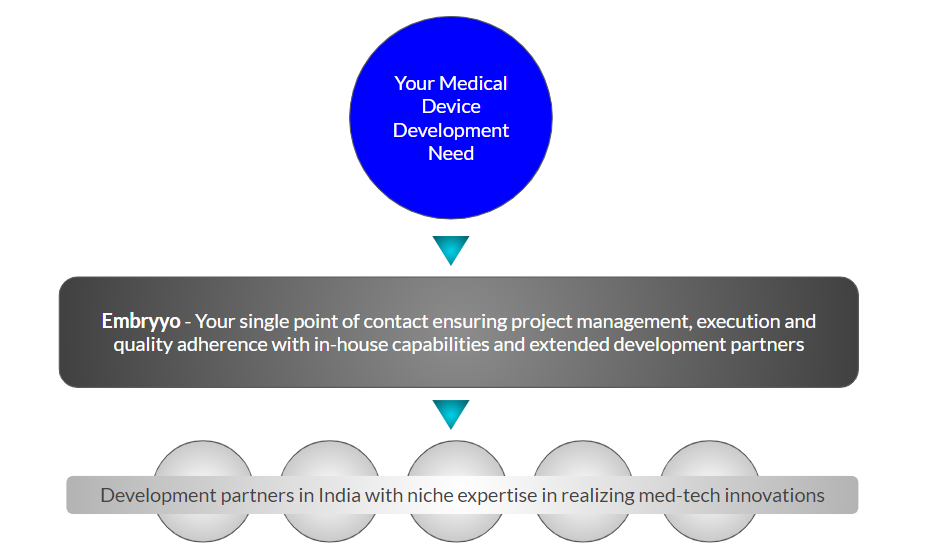
Embryyo: Pioneering Next-Generation Medical Device Engineering
In the ever-evolving landscape of medical technology, India emerges as a beacon of innovation and efficiency. For global medical device manufacturers, innovators, and entrepreneurs, the quest for a reliable, quality-driven, and comprehensive service partner ends with Embryyo. At Embryyo, we specialize in transforming your visionary medical device concepts into tangible, market-ready solutions.
The 'India' advantage
India is emerging as one of the leaders in global medical device engineering and manufacturing, leveraging its vast engineering talent and cost-effective, innovative approach. Producing over 1.5 million engineering graduates annually, India offers significant R&D capabilities at 25-30% lower costs than Western countries, a major draw for outsourcing.
The Indian biotech and medical device sector, demonstrating a robust Compound Annual Growth Rate (CAGR) of over 16.8% between 2015 and 2020, reflects the country's dynamic, innovation-led growth. This expansion is strongly supported by government-led initiatives such as 'Make in India', 'Med-Tech Mitra' and the establishment of specialized 'Med-Tech Parks', which provide state-of-the-art infrastructure and attractive incentives, further enhancing India's global appeal as a med-tech innovation and R&D hub. The size of the Indian medical devices market is estimated at $11 Bn, and is expected to grow to $50 Bn by 2025 and the sector has been growing steadily at a CAGR of 15% over the last 3 years.
The sector's commitment to quality is evident in its 665+ FDA-registered facilities nationwide, aligning with international standards. This commitment to quality is mirrored in the sector's export performance, achieving a 17% growth in FY 2022-23, reaching key markets like the USA and Germany, as per the Association of Indian Medical Devices Industry (AiMED). Moreover, global corporations like Medtronic, Boston Scientific and others are investing in India, establishing R&D centers and leveraging local expertise for global product development, underscoring India's growing prominence in the medical technology field.
Being deeply embedded in the Indian med-tech ecosystem, Embryyo is well-positioned to harness the 'India advantage' to deliver quality, innovation, and efficiency in all projects.
The Indian biotech and medical device sector, demonstrating a robust Compound Annual Growth Rate (CAGR) of over 16.8% between 2015 and 2020, reflects the country's dynamic, innovation-led growth. This expansion is strongly supported by government-led initiatives such as 'Make in India', 'Med-Tech Mitra' and the establishment of specialized 'Med-Tech Parks', which provide state-of-the-art infrastructure and attractive incentives, further enhancing India's global appeal as a med-tech innovation and R&D hub. The size of the Indian medical devices market is estimated at $11 Bn, and is expected to grow to $50 Bn by 2025 and the sector has been growing steadily at a CAGR of 15% over the last 3 years.
The sector's commitment to quality is evident in its 665+ FDA-registered facilities nationwide, aligning with international standards. This commitment to quality is mirrored in the sector's export performance, achieving a 17% growth in FY 2022-23, reaching key markets like the USA and Germany, as per the Association of Indian Medical Devices Industry (AiMED). Moreover, global corporations like Medtronic, Boston Scientific and others are investing in India, establishing R&D centers and leveraging local expertise for global product development, underscoring India's growing prominence in the medical technology field.
Being deeply embedded in the Indian med-tech ecosystem, Embryyo is well-positioned to harness the 'India advantage' to deliver quality, innovation, and efficiency in all projects.
Why Embryyo? Our value add -
|
Med-Tech Product Realization Capabilities in Conjunction with Our Extended Ecosystem of 30+ Partners
|
Mechanical Design Mechanism design, analysis and synthesis | Stress and tolerance analysis | Kinematics and dynamics | Material selection | Design for assembly | Robotics and automation design | Enclosures and packaging | Design for manufacturing Embedded Systems Design Embedded hardware and firmware | lOT solutions | Sensor integrations | FPGA development | Communication protocols | Cloud connectivity | Edge devices | RTOS | Multilayer, high speed PCBs | EMI / EMC compliant designs Custom Software Development Medical device-as-a-software design | Mobile applications | Full system integrations | AI/ML integrations | Cloud computing solutions | UI/UX implementation | Data analytics | Software prototyping Industrial Design UI / UX | Design thinking | Form visualization | Mockups | Graphics design | Human factors research Engineering Simulations Computational fluid dynamics | Finite element modeling Microfluidics Custom microfluidic chip fabrication | Fluidic instrumentation | Mold design | Variations in substrate material Electrochemistry Gold wire bonding | Aluminum wire bonding | Active and passive laser trimming | Thick film technology and digital electronics Thin-Film Coatings Thin film coating equipment | Advanced plasma processing equipment | Magnetron sputtering | Arc deposition | Plasma ion nitriding | Pulsed power supplies | Corona poling Optics Design and Assembly Self-centering & gimbal optical mounts | Threshold laser coatings | AR coatings | Beam splitters | Optical filter | High accuracy dimensional tolerances <5 micron | Multi-layer anti reflection coatings Polymers Single use assemblies | Silicone-based products like tubings | Silicone sleeves | Customized silicone products Balloons | Catheters | Implantables Stent laser cutting | Drug coating | Balloon forming | Catheter extrusion | Wire braiding | Laser & RF welding | Guide catheter assembly | Sterilization | Braided & coiled tubing manufacturing | Coiled reinforcing & coatings | Hybrid reinforcing | Tube extrusions | Precision micro-welding | Micro-hole punching Limited Batch Prototyping CNC machining | Sheet metal | Injection molding | Tool making | Casting | Electronics packaging | PCB manufacturing, assembly and testing Additive Manufacturing Precision and complex parts | Layer-by-Layer manufacturing | Post-processing | CAD/CAM integrations | Custom tooling and fixtures | Bioprinting Assembly Automation Assembly automation using linear and rotary indexing systems | Vision system sensing | Electrical grippers & electro- pneumatic pick and place systems | Linear drive technology with continuous motion Contract Manufacturing MDSAP, ISO 13485:2016, FDA (21 CFR 820) compliant facility | Supply chain management | Electronics & mechanical manufacturing | Sterilization| Packaging & Labeling Pre-Clinical Testing Biocompatibility | Toxicity | Hemocompatibility | In-vitro and animal testing | Durability and fatigue testing | Simulated use testing | Material characterization | Electrical safety | Accelerated life testing | Custom test method development |